Plasma Fractionation
Plasma Fractionation: Advancing Life-Saving Therapies
What is Plasma Fractionation?
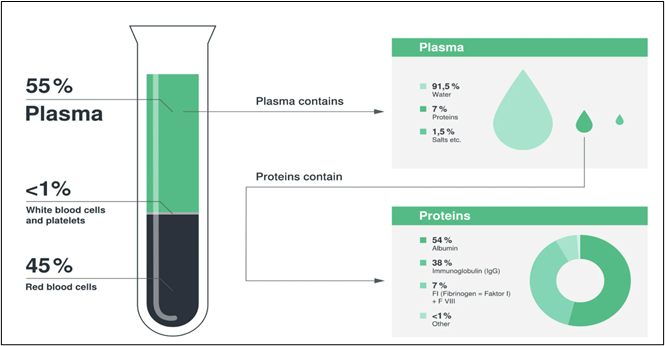
Plasma fractionation is a specialized biotechnological process used to extract vital therapeutic proteins from human plasma, the liquid component of blood. These proteins include:
- Human Serum Albumin (HSA)
- Immunoglobulins (IgG, IgM, IgA)
- Coagulation Factors (Factor VIII, Factor IX)
- Protease Inhibitors
- Monoclonal Antibodies (mAbs)
These components play a critical role in treating life-threatening and chronic medical conditions, making plasma fractionation an essential part of modern healthcare.
A Legacy of Innovation
First developed during World War II to produce serum albumin for treating shock and burns, the field has since evolved through innovations in cold ethanol fractionation, chromatographic purification, and viral inactivation methods. Today, plasma fractionation is at the intersection of biotechnology, transfusion medicine, and pharmaceutical manufacturing.

Applications of Plasma-Derived Medicinal Products (PDMPs)
PDMPs are life-saving biologics used in a wide range of medical applications:
- Hemophilia Treatment – Factor VIII, Factor IX
- Primary Immunodeficiency Therapies – Intravenous (IVIG) & Subcutaneous (SCIG) Immunoglobulins
- Autoimmune & Neurological Disorders – Guillain-Barré Syndrome, Myasthenia Gravis
- Post-Exposure Prophylaxis – Hyperimmune globulins for hepatitis B, rabies, tetanus
- Volume Expansion – Albumin for trauma, burns, hypoalbuminemia
- Specialty Uses – Rh(D) Immune Globulin, α1-Antitrypsin, Antithrombin III, C1-Esterase Inhibitor, Fibrin Sealants
These therapies are indispensable in managing conditions that would otherwise lead to significant morbidity or mortality.
Our Vision: World-Class Plasma Fractionation Facility
We are establishing a state-of-the-art Human Plasma-Derived Medicinal Products (HPDMP) manufacturing facility with an initial annual capacity of 250,000 liters, scalable to 500,000 liters. Our facility is designed to:
- Deliver high-purity, safe, and effective PDMPs
- Ensure regulatory compliance and GMP standards
- Support India's self-reliance in critical blood-plasma products
- Advance research in monoclonal antibodies (mAbs) and novel therapeutics
Our Process
Plasma fractionation is a multi-step, quality-controlled process, including:
- Plasma Collection and Testing
- Plasma Pooling
- Cryoprecipitation
- Cold Ethanol Fractionation & Chromatographic Purification
- Viral Inactivation and Removal
- Final Formulation, Sterile Filtration
- Storage and Distribution
This robust process ensures every product meets the highest standards of safety, purity, and clinical efficacy.
Global and Indian Market Outlook
The global plasma fractionation market was valued at USD 27.1 billion in 2022 and is projected to reach USD 51.8 billion by 2032, growing at a CAGR of 6.7%. The Asia-Pacific region is expected to be the fastest-growing market.
In India, the plasma fractionation market was valued at USD 331.7 million in 2020, with projected growth to USD 470.6 million by 2030 at a CAGR of 4.2%. This highlights the growing need for self-sustaining plasma infrastructure and locally manufactured PDMPs.
Why It Matters
By unlocking the therapeutic potential of human plasma, we are empowering healthcare systems to treat rare and complex diseases more effectively. Plasma fractionation saves millions of lives globally and enhances the quality of life for patients facing otherwise debilitating conditions.
Join Us in Transforming Healthcare
We are committed to delivering life-saving innovations through science, safety, and sustainability. Whether you're a partner, clinician, policymaker, or patient advocate, we invite you to be part of a future where biological therapies transform human health.

