In-vitro Diagnostic
In Vitro Diagnostics (IVD) and Point-of-Care Testing (PoCT) Devices
In Vitro Diagnostics (IVD) and Point-of-Care Testing (PoCT) devices are revolutionizing modern healthcare by enabling fast, accurate, and accessible diagnostic testing. IVD refers to tests performed on samples such as blood, urine, or tissue that have been taken from the human body, providing critical insights into a patient’s health status. PoCT devices, a subset of IVD, are designed for use at or near the site of patient care, delivering rapid results outside of traditional laboratory settings.
Lifenity Group is committed towards providing the cutting-edge technology and solutions that are patient friendly and affordable
Benefits of IVD and PoCT Devices
-
Early and Accurate Diagnosis
IVD and PoCT technologies facilitate early detection of diseases such as diabetes, infectious diseases, cancer, and cardiovascular conditions. This leads to timely medical intervention and improved patient outcomes. -
Early and Accurate Diagnosis
IVD and PoCT technologies facilitate early detection of diseases such as diabetes, infectious diseases, cancer, and cardiovascular conditions. This leads to timely medical intervention and improved patient outcomes. -
Rapid Turnaround Time
PoCT devices provide real-time or near-real-time results, enabling immediate clinical decisions, especially crucial in emergency or critical care settings. -
Improved Patient Convenience
Patients benefit from quicker testing procedures and results, reducing the need for multiple hospital visits and minimizing discomfort. -
Operational Efficiency
Healthcare facilities can streamline workflows, reduce laboratory workloads, and lower overall healthcare costs through decentralized testing models. -
Enhanced Accessibility
In resource-limited or remote areas, PoCT devices extend the reach of quality healthcare by providing diagnostic capabilities without the need for advanced infrastructure.
Advantages of Manufacturing IVD and PoCT Devices
-
Growing Global Demand
With the rising prevalence of chronic and infectious diseases, aging populations, and an increasing focus on preventive healthcare, the demand for IVD and PoCT devices is surging worldwide. -
Technological Innovation
Manufacturing IVD and PoCT devices offers a platform for integrating cutting-edge technologies such as microfluidics, biosensors, AI, and connectivity, fostering innovation and competitive differentiation. -
High Market Potential
The IVD and PoCT market continues to expand, driven by ongoing advancements in personalized medicine, home diagnostics, and telehealth. This creates robust commercial opportunities for manufacturers. -
Regulatory Incentives and Support
Many governments and health agencies provide incentives, streamlined regulatory pathways, and funding support to boost domestic production of diagnostic technologies. -
Scalability and Customization
Manufacturers can tailor IVD and PoCT solutions to specific healthcare needs, regions, or patient demographics, allowing for scalable and versatile product offerings.
The development and manufacturing of In Vitro Diagnostics and Point-of-Care Testing devices represent a strategic and impactful contribution of Lienity Group to global healthcare. By enabling faster, more accurate, and accessible diagnostics, these technologies empower healthcare providers and improve patient care. By, investing in this sector offers not only strong economic returns but also the opportunity to drive meaningful change in public health.
Devices Enlisted
Lifenity Multi-Use Dialysers
- 5-6 time reuse to save cost
- Carefully Curated Fibre to provide absolute sterilisation
- Unique PP Design

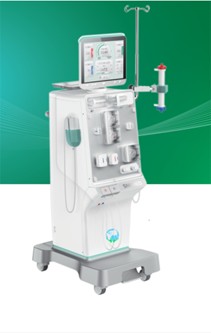
Lifenity Haemodialysis Machine
- Ultra pure Dialysis Treatment
- Online- Hdf Treatment
- Modular Distributed Design
- Precise Ultrafication
- Mix Dilutions
- Simpler Maintenance
- User Friendly UI
- CE Certified
HIV detection by Urine
The HIV 1.2 Rapid Test (Urine) is a rapid chromatographic immunoassay for the qualitative detection of antibodies to Human Immunodeficiency Virus (HIV) type 1and type 2 in urine to aid in the diagnosis of HIV infection

